PhD Students (ENB)

Sayonika Chakraborty
Email: Sayonika.Chakraborty@ur.de
M.Sc.: Pune University, India
B.Sc.: B.J.B Autonomous College, Bhubaneswar, India
Internship: Indian Institute of Science, Bengaluru, India
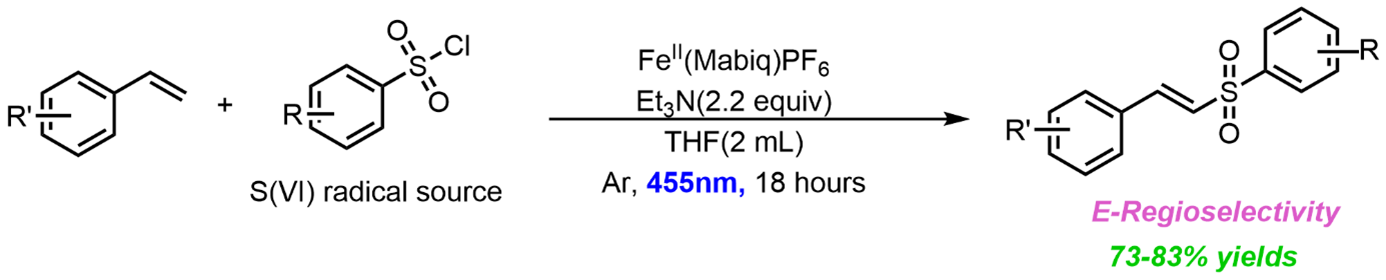
Research Project: Exploring the Photocatalytic Activity of Iron-Mabiq Complexes towards C-S bond formation
Supervisor: Prof. Dr. Corinna Hess (UR)
Co-Supervisor: Prof. Dr. Oliver Reiser (UR)
Industrial Mentor: Prof. Dr. Magnus Johansson (AstraZeneca)
Project abstract
Visible-light-mediated photoredox catalysis by light-harvesting molecules that facilitate an electron transfer mechanism is being explored in various ways. So far, Iron mediated photoredox catalysis has not been explored to its full potential. Low excited state lifetime is a major drawback for Fe-photoredox catalysis. One of the factors to overcome this draw back would be to have a stable ligand design which tunes the redox properties as well as stabilizes the excited state lifetime. We chose light-driven activation of Sulfonyl chloride electrophile into S(VI) radicals for alkene ligation as the benchmark reaction to explore the photoactive properties of Fe-Mabiq.

Luis Antonio Coelho
M.Sc.: Universidad Simón Bolívar, Caracas, Venezuela
B.Sc.: Universidad Simón Bolívar - Caracas, Venezuela
Internship: Venezuelan Institute for Scientific Research (IVIC), Miranda, Venezuela; University of Bath, England, UK
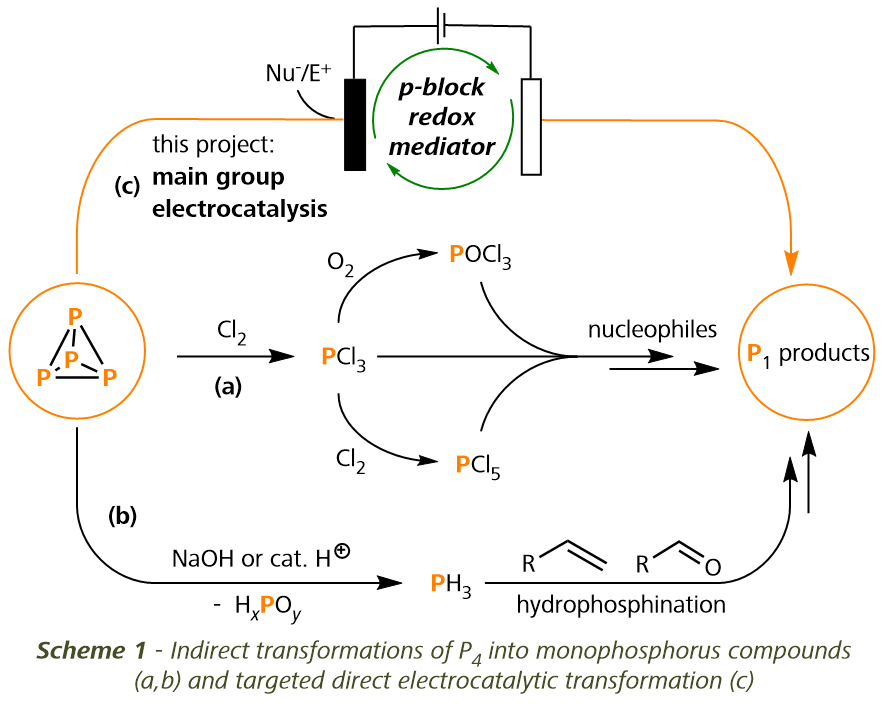
Research Project: Electrocatalytic functionalization of white phosphorus
Supervisor: Prof. Dr. Robert Wolf (UR)
Co-Supervisor: Dr. Daniel Scott (University of Bath, UK)
Industrial Mentor: Dr. Carolin Limburg (BASF)
Publications:
- T. M. Horsley Downie, A. Velić, L. A. Coelho, R. Wolf, D. J. Scott, Closing the Loop: Low-Waste Phosphorus Functionalization Enabled by Simple Disulfides, ChemSusChem 2024, e202401895.
Project abstract
White phosphorus (P4) serves as the primary starting material for the preparation of nearly all commercially significant monophosphorus compounds.
Currently, the most widely used methods to synthesize P1 products depend on the oxidation and disproportionation of P4, which produce intermediates
such as phosphorus trichloride (PCl3) and phosphine (PH3) (Scheme 1a, b).[1] However, the dependence on multiple reaction steps and the generation
of copious amounts of chemical waste have long raised concerns and highlighted the need for the development of safer and more environmentally friendly
alternatives.[2] We are developing straightforward strategies for preparing monophosphorus products directly from P4. Our methods involve electrocatalytic
systems mediated by abundant and recyclable main-group compounds (Scheme 1c). Electrical energy input is used to drive thermodynamically uphill transformations,
contrasting with the traditional approach of employing high-energy intermediates and stochiometric reagents
References:
[1] a) H. Diskowski, T. Hofmann, “Phosphorus” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley, Weinheim, 2022;
b) J. Svara, N. Weferling, T. Hofmann, “Phosphorus compounds, organic” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley, Weinheim, 2006.
[2] a) S. Beijer, J. C. Slootweg, “Sustainable Phosphorus” in Encyclopedia of Inorganic and Bioinorganic Chemistry, Wiley, Weinheim, 2021;
b) D. J. Scott, Angew. Chem. Int. Ed. 2022, 61, e202205019.

Léa Di Luzio
Email: lea.di-luzio@tum.de
M.Sc.: University of Geneva, Switzerland
B.Sc.: University of Geneva, Switzerland
M.Sc. Internship: Bioinorganic chemistry, Pr. Ross Milton, University of Geneva, Switzerland
M.Sc. Thesis: New process development, Firmenich SA, Switzerland
Research Project: Artificial copper enzymes for photoredox catalysis
Supervisor: Prof. Dr. Cathleen Zeymer (TUM)
Co-Supervisor: Prof. Oliver Reiser (UR)
Industrial Mentor: Dr. Erika Tassano (Novartis)
Project abstract
Copper (I/II) catalysts are currently of great interest for applications in visible light photoredox chemistry, as they offer an alternative to precious metals such as ruthenium (II) or iridium (III) and allow to control the stereochemistry of the reactions. Therefore, we are working on introducing non canonical amino acids (such as BpyAla) in de novo protein scaffolds, in order to create a chiral environment to perform various stereoselective photoredox reactions (Atom transfer radical additions to olefins, oxo-azidation, oxo-amination…).

Jonas Düker
Email: Jonas.Dueker@ur.de
M.Sc.: University of Regensburg, Germany
B.Sc.: University of Regensburg, Germany
Internship: Sigman Group, University of Utah, USA
Research Project:
Supervisor: Prof. Dr. Burkhard König (UR)
Co-Supervisor: Prof. Dr. Robert Wolf (UR)
Industrial Mentor: Dr. Julius Hillenbrand (Bayer)
Publications:
- I. Ghosh, N. Shlapakov, T. A. Karl, J. Düker, M. Nikitin, J. V. Burykina, V. P. Ananikov, B. König, General cross-coupling reactions with adaptive dynamic homogeneous catalysis, Nature 2023, 619, 87-93.
- J. Düker, I. Ghosh, B. König, Sequential One-Pot (Het)arene Thioetherification and Amination with Nickel and Visible Light, ACS Catal. 2023, 13, 20, 13618–13625.
- J. Düker, M. Philipp, T. Lentner, J. A. Gadge, J. E. A. Lavarda, R. M. Gschwind, M. S. Sigman, I. Ghosh, B. König, Cross-Coupling Reactions with Nickel, Visible Light, and tert-Butylamine as a Bifunctional Additive, ACS Catal. 2025, 15, 2, 817–827.
- J. Düker, N. Petersen, N. Richter, P. Feuerer, T. Faber, N. Hölter, N. Kehl, J. Oboril, J. Strippel, A. Gröer, N. Guimond, S. J. Kaldas, M. Lübbesmeyer, G. Volpin, B. König, J. Hillenbrand, Room-Temperature Copper Cross-Coupling Reactions of Anilines with Aryl Bromides, Org. Lett. 2025, 27, 22, 5566–5571.
Project abstract
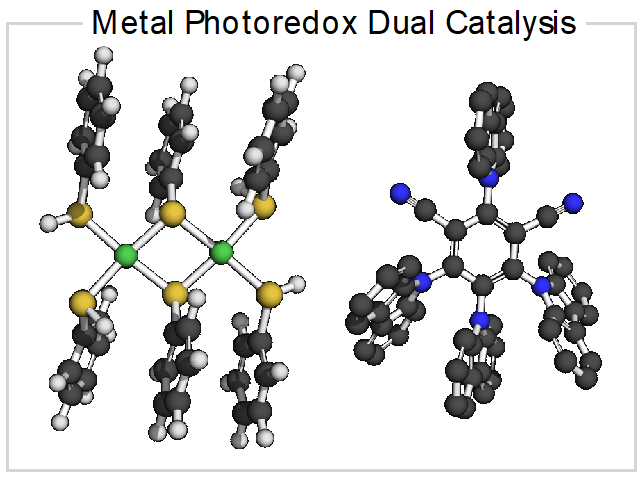
Development of metal photoredox dual catalytic systems and their mechanistic investigation to address limitations in kinetically challenging bond activations. Moreover, structure-activity relationships will be investigated with the aid of data science tools.

Daniela Fritsch
Email: Daniela.Fritsch@ur.de
M.Sc.: University of Regensburg, Germany
B.Sc.: University of Regensburg, Germany
Research Project: Light-induced deoxygenations of mono- and polyols
Supervisor: Prof. Dr. Alexander Breder (UR)
Co-Supervisor: Prof. Dr. Oliver Reiser (UR)
Industrial Mentor: Dr. Christian Gampe (Genentech, USA)
Publications:
- S. Park, A. K. Dutta, C. Allacher, A. Abramov, P. Dullinger, K. Kuzmanoska, D. Fritsch, P. Hitzfeld, D. Horinek, J. Rehbein, P. Nuernberger, R. Gschwind, A. Breder, Hydrogen-Bond-Modulated Nucleofugality of SeIII Species to Enable Photoredox-Catalytic Semipinacol Manifolds, Angew. Chem. Int. Ed. 2022, e202208611.
Project abstract
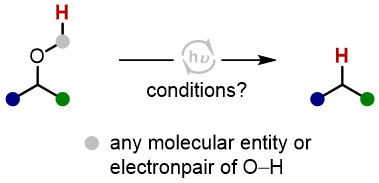
Deoxygenation reactions of aliphatic, non-benzylic alcohols and polyols represent an important subcategory of
defunctionalization reactions, utilized for late-stage structural editing of complex molecules. Strategies for the
activation of carbinolic C–O bonds have been amply developed, which proceed either through stoichiometric pre-activation
of the O-atom to increase its nucleofugality, by catalytic C–O bond activation with – predominantly precious – transitions
metals, or by C–O bond weaking via radical pathways.[1] While in all of these methods the H atom usually stems from a separate
(stoichiometric) reagent, we are investigating a complementary, light-driven catalytic protocol based on a "hydrogen atom
shuffling mechanism", in which the H atom of the newly formed C–H bond derives from the OH group of a substrate molecule.
[1] K. Anwar, K. Merkens, F. J. A. Troyano, A. Gómez-Suárez Eur. J. Org. Chem. 2022, e202200330.

Johannes Hofer
Email: Johannes.Hofer@tum.de
M.Sc.: Technische Universität München (TUM)
B.Sc.: Technische Universität München (TUM)
Research Project: Activation of CH-bonds through photocatalytic processes
Supervisor: Prof. Dr. Thorsten Bach (TUM)
Co-Supervisor: Prof. Dr. Cathleen Zeymer (TUM)
Industrial Mentor: Dr. Richard Brimioulle (Merck)
Project abstract
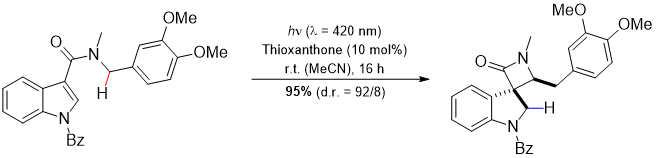
Investigation of photocatalytic reactions, which enable intra- or intermolecular CH-bond-activation by an excited chromophore.

Mattia Lepori
Email: Mattia.Lepori@ur.de
M.Sc.: University of Pisa
B.Sc.: University of Pisa
Internship: University of Amsterdam, Netherlands
Research Project: Photo- and Electrochemical Multicomponent Strategies to Rapidly Build Molecular
Complexity in Batch and Continuous Flow
Supervisor: Prof. Dr. Joshua Barham (UR, University of Strathclyde)
Co-Supervisor: Prof. Dr. Timothy Noel (University of Amsterdam, Netherlands)
Industrial Mentor: Blandine McKay (GSK)
Project abstract
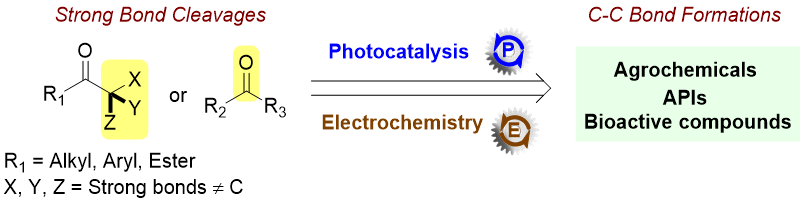
Development of new photo and/or electrochemical methods of strong bonds cleavages and C-C bond formations toward the synthesis of pharmaceutical, agrochemical and bioactive molecules.

Maximilian Philipp
Email: Maximilian.Philipp@ur.de
M.Sc.: University of Regensburg
B.Sc.: University of Ulm
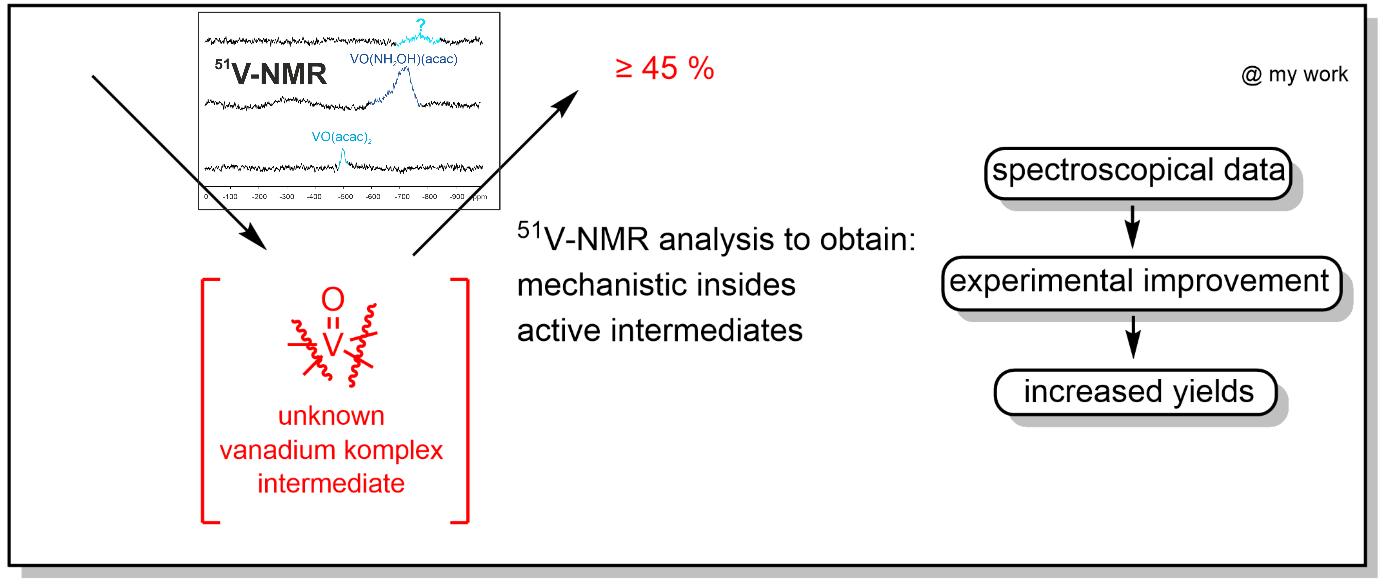
Research Project: Unraveling Catalytic Dynamics: NMR Insights into Metal-Catalyzed Photocatalytic Transformations
Supervisor: Prof. Dr. Ruth Gschwind (UR)
Co-Supervisor: Prof. Dr. Burkhard König (UR)
Industrial Mentor: Prof. Dr. Bernd Schäfer (BASF SE)
Project abstract
In collaboration with the group of Prof. König a vanadium co-catalysed photochemical reaction is investigated via 51V-NMR spectroscopy. This novel approach should give insights in the reaction mechanism and active intermediates to help further develop and improve the reaction experimentally.

Vanshika Raheja
Email: Vanshika.Raheja@ur.de
M.Sc.: University of Delhi
B.Sc.: University of Delhi
Internship: Indian Institute of Science and Technology Delhi
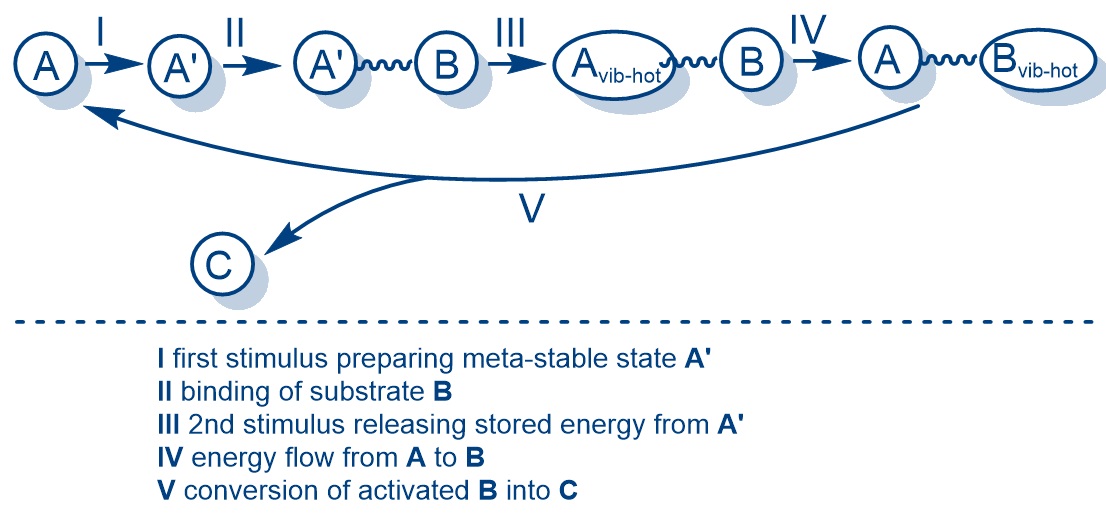
Research Project: Strain-release activation of organic transformations harnessing quadricyclane intermediates as molecular energy storage devices
Supervisor: Prof. Dr. Julia Rehbein (UR)
Co-Supervisor: Prof. Dr. Oliver Reiser (UR)
Industrial Mentor: Dr. Christian Gampe (Genentech)
Project abstract
Renewable and clean energy sources are a highly active area of research. Molecular solar-thermal energy storage systems, which are based on molecular switches, reversibly convert solar energy into chemical energy. In these systems, photochromic molecules are converted by irradiation into their respective metastable photoisomers. The energy stored in this way can later be released on demand, either through thermal or catalytic activation. Norbornadiene–quadricyclane (NBD/QC) photoisomers are promising candidates for energy storage, as quadricyclane is a high-energy isomer with a double cyclopropane ring structure. The stored energy can be released using a catalyst—thermally, photochemically, or under electrochemical conditions. Approximately 1000 kJ/kg can be stored in the QC framework. Contrary to previous research, we are focusing on the fundamental question of whether the strain-release energy from QC (A´) to NBD (A) can be channeled directly via hydrogen bonds to reactants (B) through vibrational mode coupling, rather than simply dissipating as heat in solution.

Arezoo Tanbakoochian
Email: Arezoo.Tanbakoochian@ur.de
M.Sc.: University of Teheran, Iran
B.Sc.: University of Beheshti, Iran
Internship: University of Heidelberg
Research Project: Photo- and Electro-catalyzed Synthesis of Biofuels
Supervisor: Prof. Dr. Oliver Reiser (UR)
Co-Supervisor: Prof. Davide Ravelli (U Pavia)
Industrial Mentor: Dr. Matthias Schmalzbauer (BASF)
Publications:
- A. Tanbakouchian, E. Kianmehr, Palladium-catalyzed regioselective direct C-H bond alkoxycarbonylation of 2-arylimidazo[1,2-a]pyridines, New J. Chem. 2021,45, 12145-12149.
- E. Kianmehr, Mohammad R. Falahat, A. Tanbakouchian, Copper-Mediated Direct Cyanata-tion of Benzamides: A New Approach to the Synthesis of Quinazolinediones, Eur. J. Org. Chem., 2020, 2020, 708.

Thomas Tiefel
Email: Thomas.Tiefel@ur.de
M.Sc.: University of Regensburg
B.Sc.: University of Regensburg
Internship: Zhang group, Boston College, USA
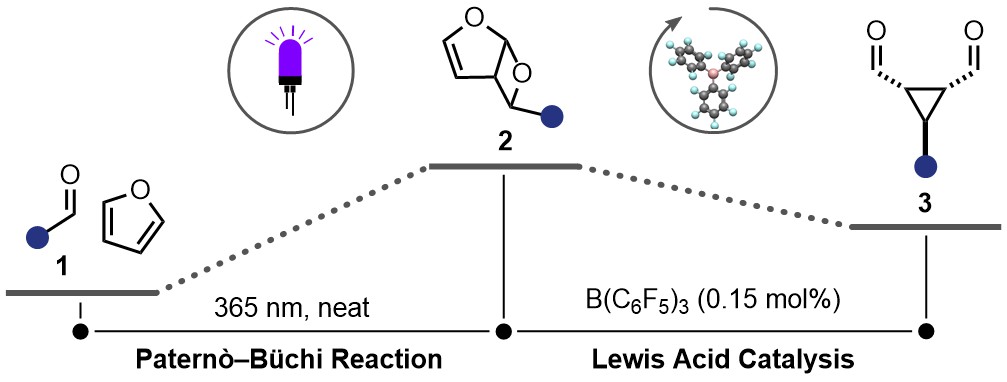
Research Project: Synthesis of Thermodynamically Challenging Cyclopropanes Through Furan Dearomatizations
Supervisor: Prof. Dr. Oliver Reiser (UR)
Co-Supervisor: Prof. Dr. Julia Rehbein (UR)
Industrial Mentor: Dr. Pablo Gabriel (Novartis)
Project abstract
Cyclopropanes are highly interesting target molecules, due to their significance in medicinal chemistry and a structure highly activated by ring-strain. While furans hold significant promise in the transition to biobased, sustainable production pathways, their conversion into more valuable fine chemicals often requires dearomatization, needing to overcome approximately 15 kcal/mol of resonance stabilization energy. Therefore, using light energy as energy input, we are developing endergonic syntheses for thermodynamically challenging cyclopropanes.